First published randomized controlled trial for market-leading implant demonstrates impressive outcomes for rotator cuff tears
Smith+Nephew (LSE:SN, NYSE: SNN), the global medical technology company, today announces standout results for its REGENETEN Bioinductive Implant from a recently completed randomized controlled trial (RCT). Published online in Arthroscopy in December, the report concluded that at one-year, medium and large full-thickness rotator cuff tears repaired and augmented with the REGENETEN Bioinductive Implant had better tendon healing compared to the standard of care,1 as evidenced by:
- A significantly lower re-tear rate (8.3 vs 25.8%; p=0.01)
- A three times lower risk of re-tear (RR=0.32; 95% CI:0.13–0.83)
- No difference in the number of serious or minor complications
The blinded, multi-centre RCT compared the healing rate of full-thickness rotator cuff tears repaired with and without augmentation with the REGENETEN Bioinductive Implant. In total, 124 patients with reparable medium and large (1–4cm) full-thickness posterosuperior rotator cuff tears were randomized to receive either an arthroscopic transosseous equivalent double-row rotator cuff repair or the same repair augmented with the REGENETEN Bioinductive Implant.
Dr. Miguel Ruiz Ibán, Head of the Shoulder and Elbow Unit at Hospital Universitario Ramón y Cajal in Madrid, Spain, and Principal Investigator for the study commented on the results, “Rotator cuff repair is traditionally associated with re-tear rates in excess of 20%, presenting a challenge for surgeons and patients alike. This RCT demonstrates that the REGENETEN Bioinductive Implant substantially and significantly reduced this risk. With no increase in complications seen in our study, these findings support the use of the implant to reduce re-tear rates in the population studied.”
With more than 100,000 procedures completed globally since its introduction in 2014, the REGENETEN Bioinductive Implant has had a transformative impact, offering a better solution for more than 1 million2 people having surgery for a rotator cuff tear each year. The collagen-based implant supports the body’s natural healing response to facilitate the formation of new tendon-like tissue to biologically augment the existing tendon and change the course of rotator cuff tear progression.1,3-8
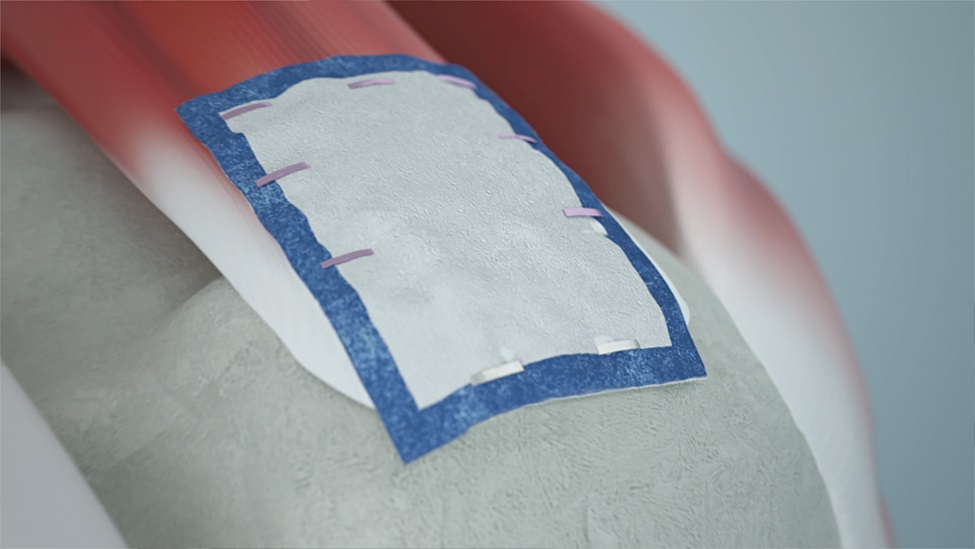
Smith+Nephew's REGENETEN Bioinductive Implant
“Smith+Nephew is committed to proving the value of our technology through support for clinical evidence generation,” said Christie Van Geffen, SVP Sports Medicine Global Marketing at Smith+Nephew. “We are pleased and excited by the opportunity presented by this evidence to drive adoption of, and access to, the REGENETEN Bioinductive Implant. Our goal is to help surgeons improve the care of many more patients with rotator cuff injuries.”
The REGENETEN Bioinductive Implant is part of Smith+Nephew’s comprehensive Advanced Healing Solutions portfolio – redefining biological healing in rotator cuff repair.
To learn more about the REGENETEN Bioinductive Implant, please click here.
- ends -
Media Enquiries
David Snyder
Smith+Nephew
+1 978-749-1440
david.snyder@smith-nephew.com
References
- Ruiz Ibán MA, Navlet MG, Marco SM, et al. Augmentation of a transosseous equivalent repair in posterosuperior non-acute rotator cuff tears with a bioinductive collagen implant decreases the re-tear rate at one year. A randomised controlled trial. Arthroscopy. Published online 12/27/2023.
- iData Research. Rotator cuff repair and reconstruction market size, share and trends analysis (2023). Available at: https://idataresearch.com/product/rotator-cuff-repair-reconstruction-market-size-share-and-trends-analysis-global-2023-2029-medsuite-includes-grafts-allografts-xenograft-synthetic-and-1-more/#. Accessed December 19, 2023.
- Bokor DJ, Sonnabend D, Deady L, et al. Evidence of healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a collagen implant: a 2-year MRI follow-up. Muscles, Ligaments Tendons J. 2016;6(1):16-25.
- Schlegel TF, Abrams JS, Bushnell BD, Brock JL, Ho CP. Radiologic and clinical evaluation of a bioabsorbable collagen implant to treat partial-thickness tears: a prospective multicenter study. J Shoulder Elbow Surg. 2018 27(2):242-251.
- Van Kampen C, Arnoczky S, Parks P, et al. Tissue-engineered augmentation of a rotator cuff tendon using a reconstituted collagen scaffold: a histological evaluation in sheep. Muscles Ligaments Tendons J. 2013;3(3):229-235.
- Arnoczky SP, Bishai SK, Schofield B, et al. Histologic Evaluation of Biopsy Specimens Obtained After Rotator Cuff Repair Augmented With a Highly Porous Collagen Implant. Arthroscopy. 2017;33(2):278-283
- Bokor DJ, Sonnabend DH, Deady L, et al. Healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a highly porous collagen implant: a 5-year clinical and MRI follow-up. Muscles, Ligaments Tendons J. 2019;9(3):338-347.
- McElvany MD, McGoldrick E, Gee AO, Neradilek MB, Matsen FA, 3rd. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43(2):491-500.
About Smith+Nephew
Smith+Nephew is a portfolio medical technology company focused on the repair, regeneration and replacement of soft and hard tissue. We exist to restore people’s bodies and their self-belief by using technology to take the limits off living. We call this purpose ‘Life Unlimited’. Our 19,000 employees deliver this mission every day, making a difference to patients’ lives through the excellence of our product portfolio, and the invention and application of new technologies across our three global business units of Orthopaedics, Sports Medicine & ENT and Advanced Wound Management.
Founded in Hull, UK, in 1856, we now operate in more than 100 countries, and generated annual sales of $5.2 billion in 2022. Smith+Nephew is a constituent of the FTSE100 (LSE:SN, NYSE: SNN). The terms ‘Group’ and ‘Smith+Nephew’ are used to refer to Smith & Nephew plc and its consolidated subsidiaries, unless the context requires otherwise.
For more information about Smith+Nephew, please visit www.smith-nephew.com and follow us on X, LinkedIn, Instagram or Facebook.
Forward-looking Statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading margins, market trends and our product pipeline are forward-looking statements. Phrases such as "aim", "plan", "intend", "anticipate", "well-placed", "believe", "estimate", "expect", "target", "consider" and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith+Nephew, these factors include: risks related to the impact of Covid, such as the depth and longevity of its impact, government actions and other restrictive measures taken in response, material delays and cancellations of elective procedures, reduced procedure capacity at medical facilities, restricted access for sales representatives to medical facilities, or our ability to execute business continuity plans as a result of Covid; economic and financial conditions in the markets we serve, especially those affecting healthcare providers, payers and customers (including, without limitation, as a result of Covid); price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal and financial compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers (including, without limitation, as a result of Covid); competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; relationships with healthcare professionals; reliance on information technology and cybersecurity; disruptions due to natural disasters, weather and climate change related events; changes in customer and other stakeholder sustainability expectations; changes in taxation regulations; effects of foreign exchange volatility; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith+Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith+Nephew's most recent annual report on Form 20-F, which is available on the SEC’s website at www. sec.gov, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith+Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith+Nephew are qualified by this caution. Smith+Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith+Nephew's expectations.
◊ Trademark of Smith+Nephew. Certain marks registered in US Patent and Trademark Office.
Smith+Nephew's REGENETEN Bioinductive Implant
Attachment





