IRVINE, Calif., Oct. 28, 2025 (GLOBE NEWSWIRE) -- Targeted Genomics LLC, developer of GlutenID, the only U.S. Food and Drug Administration (FDA) cleared test for celiac genetics, today announced the launch of their consumer-initiated laboratory testing service, CeliacDx designed to address the hidden epidemic of undiagnosed celiac disease.
Celiac disease is the most common intestinal autoimmune disorder worldwide with an estimated 1 in a hundred people in the US affected. However, only about 30% of the estimated 3 million Americans with celiac disease have been properly diagnosed. https://targeted-genomics.com/what-is-celiac-disease/ Undiagnosed celiac disease can lead to serious long term complications including nutrient deficiencies, other autoimmune diseases, and small bowel cancer.
CeliacDx Testing Service addresses the problem of undiagnosed celiac disease by providing consumers with direct access to laboratory testing including expert pathology interpretation of results which can be shared with their healthcare provider.
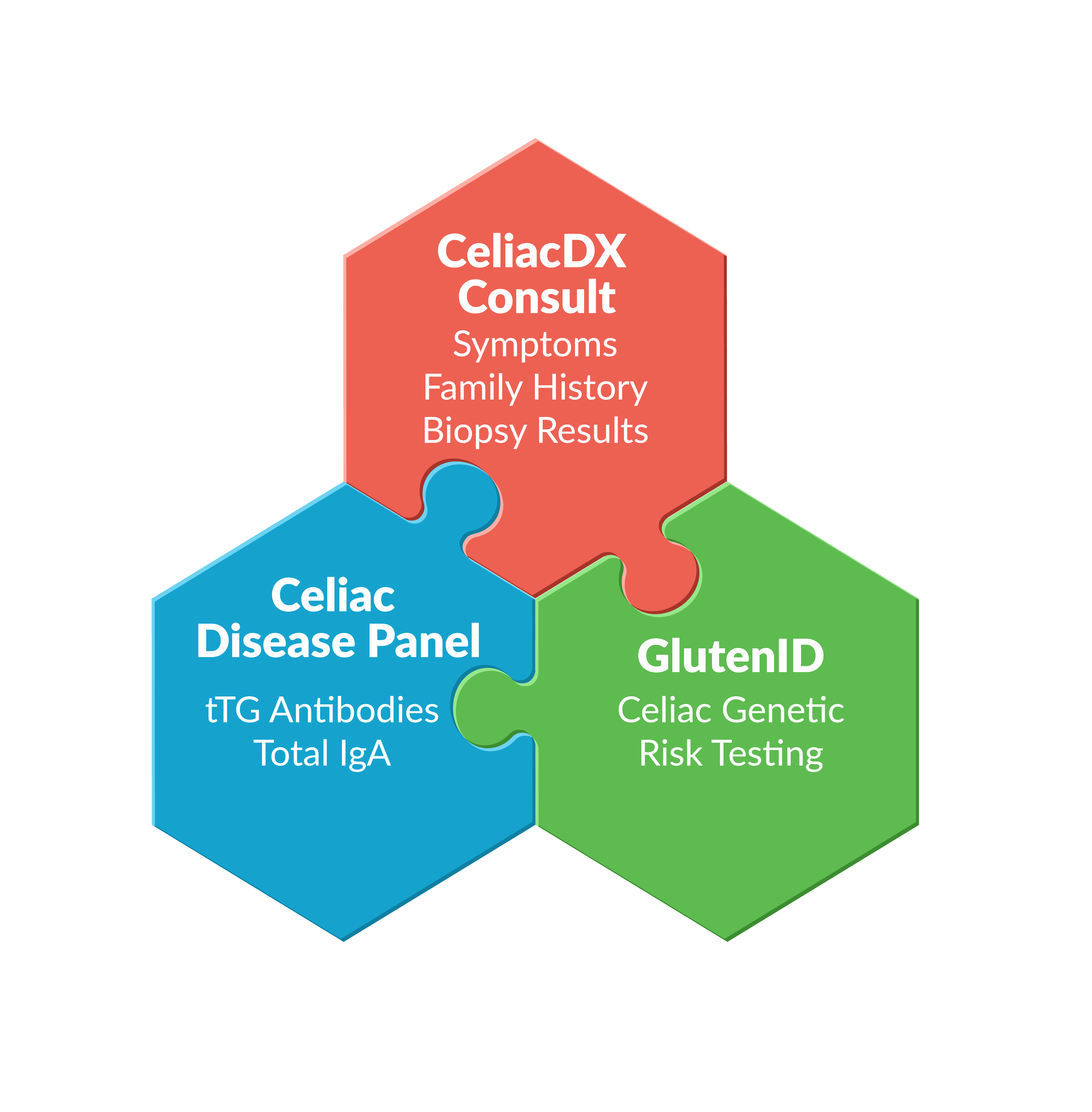
CeliacDx Testing includes three components:
- The FDA cleared GlutenID celiac genetic risk test which uses an at-home collected saliva sample to analyze genetic variants associated with increased risk for celiac disease. Negative genetic results are associated with close to 0% lifetime celiac disease risk making further celiac testing unnecessary.
- The Celiac Disease Panel for celiac antibodies which is only recommended for individuals whose GlutenID results show increased genetic risk for celiac disease.
- The CeliacDx Consult providing pathology review of antibody test results and a questionnaire for gathering data about symptoms, family, history, dietary gluten intake, and previous celiac testing results including small intestinal biopsy.
After receiving their CeliacDx Consult, users are encouraged to share the results with a licensed healthcare provider before making any changes to their diet or lifestyle.
“Time from onset of symptoms to receipt of an accurate celiac disease diagnosis can take years due to lack of access to celiac testing, and misinterpretation of test results. The CeliacDx Testing Service has great potential to accelerate the celiac diagnostic process by positively identifying those who are at genetic risk, and preventing costly, unnecessary testing for those who are not,” said Shelly Gunn MD, PhD, Founder and Medical Director at Targeted Genomics. “Our goal for CeliacDx Testing is to assemble the diagnostic puzzle pieces from consumer-initiated testing into a complete picture which aids healthcare providers in making a timely diagnosis or recommending further testing.”
The CeliacDx offering marks the company’s expansion into additional celiac testing options and furthers the Targeted Genomics mission to provide wellness-seeking consumers direct access to their celiac risk information without a prescription. The GlutenID test, Celiac Disease Panel, and CeliacDx Consult can be directly ordered from the Company website Targeted-Genomics.com
About Targeted Genomics
Targeted Genomics is a family-owned company committed to the design and development of clinical laboratory testing for wellness.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/64600377-d014-411e-aa9c-30892446db9a

Media Contact: Shelly Gunn MD, PhD shelly@targeted-genomics.com