
PureTech Health plc (LSE: PRTC, Nasdaq: PRTC) (“PureTech” or the “Company”), a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, today announced the initiation of its Phase 1 clinical trial of LYT-200 for the potential treatment of metastatic solid tumors that are difficult to treat and have poor survival rates. LYT-200 is one of several novel therapeutic opportunities within PureTech’s Wholly Owned Pipeline that will be discussed today at its virtual R&D Day.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20201211005082/en/
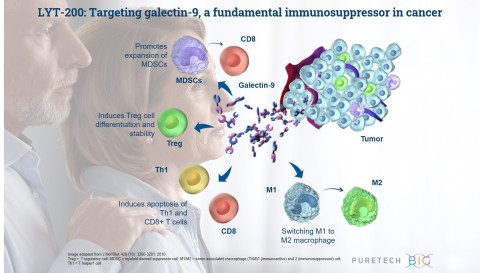
LYT-200 is a monoclonal antibody targeting a foundational immunosuppressive protein, galectin-9, for the potential treatment of solid tumors that are difficult to treat and have poor survival rates, including pancreatic ductal adenocarcinoma, colorectal cancer and cholangiocarcinoma. PureTech today announced the initiation of a Phase 1 clinical trial of LYT-200 for the potential treatment of metastatic solid tumors. LYT-200 and several novel therapeutic opportunities within PureTech’s Wholly Owned Pipeline will be discussed today during the Company’s Virtual R&D Day at 9am EST. (Photo: Business Wire)
“Each year, hundreds of thousands of people are diagnosed with solid tumors, and many will present with metastatic disease that do not respond to existing immunotherapy agents,” said Zev Wainberg, M.D., co-director of the UCLA GI Oncology Program, associate professor of medicine at UCLA and the principal investigator on PureTech’s LYT-200 trial. “By targeting galectin-9, LYT-200 is designed to block foundational immunosuppressive mechanisms that shut down the body’s natural ability to fight a number of cancers. The unique mechanism of LYT-200 holds potential across a number of solid tumor types and may enable LYT-200 to be used as a single agent, as well as in combination with checkpoint inhibitors and other anti-cancer treatments.”
LYT-200 is a clinical stage, fully human, monoclonal antibody (mAb), that is designed to target galectin-9, an immunosuppressive protein that simultaneously activates multiple immunosuppressive pathways in the tumor microenvironment and is prominently expressed in multiple difficult-to-treat cancers, including breast cancer, pancreatic and cholangiocarcinoma. It is currently being evaluated in the first stage of an adaptive Phase 1/2 trial. The primary objective of the Phase 1 portion of the trial is to assess the safety and tolerability of escalating doses of LYT-200 in order to identify a dose to carry forward into a subsequent Phase 2 trial. The Phase 1 will also assess LYT-200’s pharmacokinetic and pharmacodynamic profiles. Following the topline results, which are expected in the fourth quarter of 2021, PureTech intends to initiate the Phase 2 expansion cohort portion of the trial, which will further assess the recommended Phase 2 dose as a single agent or in combination with chemotherapy and anti-PD-1 therapy in multiple solid tumor types, including pancreatic cancer and cholangiocarcinoma.
“We are pleased to have initiated the Phase 1 part of our LYT-200 clinical trial, which is a dose-finding portion designed to assess safety and tolerability and explore preliminary signals of efficacy for LYT-200,” said Aleksandra Filipovic, M.D. PhD, head of oncology at PureTech. “We have generated compelling preclinical data to date, which we believe support the potential of LYT-200 as a new anti-cancer therapy targeting galectin-9, both as a single agent and in combination with other anti-cancer therapies.”
Dr. Zev Wainberg and Dr. Siddhartha Mukherjee, clinician and researcher at Columbia University and Pulitzer Prize-winning author of The Emperor of All Maladies and The Gene, will discuss their perspectives on the field of immuno-oncology during today’s virtual R&D Day, which will be available on the Investors section of the corporate website under Events & Presentations. To register, please sign up here.
About LYT-200
LYT-200 is a monoclonal antibody targeting a foundational immunosuppressive protein, galectin-9, for the potential treatment of solid tumors, including pancreatic ductal adenocarcinoma, colorectal cancer and cholangiocarcinoma, that are difficult to treat and have poor survival rates. PureTech has presented preclinical data demonstrating high expression of galectin-9 across breast cancer, pancreatic and cholangiocarcinoma samples and found that the highest levels of galectin-9 correlated with shorter time to disease relapse and poor survival. These data suggest that galectin-9 could be significant both as a therapeutic target for a range of cancers and as a cancer biomarker. Preclinical animal and patient-derived organoid tumor models also showed the potential efficacy of LYT-200 and the importance of galectin-9 as a target. LYT-200 is currently being evaluated in a Phase 1/2 adaptive design trial, and results from the Phase 1 portion are expected in the fourth quarter of 2021.
About PureTech’s Virtual R&D Day
The virtual R&D Day will showcase PureTech’s scientific leadership in lymphatics and related immune pathways and detail PureTech’s Wholly Owned Pipeline. Product candidates within this pipeline include LYT-100, a clinical-stage, anti-fibrotic and anti-inflammatory small molecule being advanced for the potential treatment of interstitial lung diseases and lymphedema, LYT-200, a clinical-stage monoclonal antibody targeting foundational immunosuppressive mechanisms for the potential treatment of solid tumors, and LYT-300, an oral form of allopregnanolone that is being advanced into IND-enabling studies for a range of neurological conditions. Additionally, the R&D Day will detail PureTech’s discovery platforms, including the Glyph™ technology platform, which is designed to target therapeutics to the lymphatic system and potentially enabling oral administration of natural bioactive molecules such as neurosteroids and cannabinoids, and the Orasome™ technology platform, which is designed to enable the oral administration of biotherapeutics, such as antisense oligonucleotides, short interfering RNA, mRNA, modular expression systems for therapeutic proteins, peptides and nanoparticles.
About PureTech Health
PureTech is a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, including intractable cancers, lymphatic and gastrointestinal diseases, central nervous system disorders and inflammatory and immunological diseases, among others. The Company has created a broad and deep pipeline through the expertise of its experienced research and development team and its extensive network of scientists, clinicians and industry leaders. This pipeline, which is being advanced both internally and through PureTech's Founded Entities, is comprised of 24 products and product candidates, including two that have received U.S. Food and Drug Administration (FDA) clearance and European marketing authorization. All of the underlying programs and platforms that resulted in this pipeline of product candidates were initially identified or discovered and then advanced by the PureTech team through key validation points based on the Company's unique insights into the biology of the brain, immune and gut, or BIG, systems and the interface between those systems, referred to as the BIG Axis.
For more information, visit www.puretechhealth.com or connect with us on Twitter @puretechh.
Cautionary Note Regarding Forward-Looking Statements
This press release contains statements that are or may be forward-looking statements, including statements that relate to our product candidates and approach towards addressing major diseases, future prospects, developments, and strategies. The forward-looking statements are based on current expectations and are subject to known and unknown risks and uncertainties that could cause actual results, performance and achievements to differ materially from current expectations, including, but not limited to, our expectations regarding the potential therapeutic benefits of LYT-200 in patients with solid tumors, the expected timing of results from our Phase 1 trial of LYT-120 and those risks and uncertainties described in the risk factors included in the regulatory filings for PureTech Health plc. These forward-looking statements are based on assumptions regarding the present and future business strategies of the company and the environment in which it will operate in the future. Each forward-looking statement speaks only as at the date of this press release. Except as required by law and regulatory requirements, neither the company nor any other party intends to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise.
View source version on businesswire.com: https://www.businesswire.com/news/home/20201211005082/en/
Contacts:
Allison Mead Talbot
+1 617 651 3156
amt@puretechhealth.com
