AmbioPharm, a worldwide leader in peptide API CDMO services, announces the acquisition of new, disruptive, peptide purification technology developed by YMC which leverages several of YMC’s patented1 twin-column purification processes. Twin-column chromatography or Multicolumn Countercurrent Solvent Gradient Purification (MCSGP) is a continuous chromatographic process which collects a high purity center cut fraction and internally recycles mixed impurity/product-containing side fractions all while loading fresh feed and discarding impurities (see diagram).
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230328005358/en/
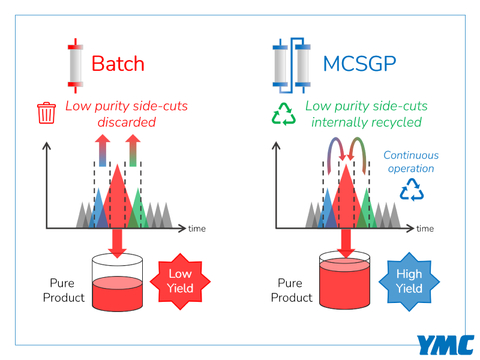
MCSGP Technology (Graphic: Business Wire)
This technology offers several advantages over traditional batch purification including:
- Higher peptide purity
- Increased yield
- Decreased solvent requirements
- Time savings
- Significant reduction of fraction collection, storage, QC, blending, and reprocessing
As a leader in peptide manufacturing and development, AmbioPharm is continuously upgrading equipment and technologies, especially those that can improve our capacity and sustainability. Using our expertise in peptide synthesis, we are able to tackle both simple and complex peptide projects with a proven history of success at research, clinical, and commercial scales.
About AmbioPharm Inc.:
AmbioPharm is a leading, innovation-driven company specializing in the development and manufacture of peptides and peptide-related products. With a comprehensive range of services, AmbioPharm produces custom products for research, clinical development, and commercial application to pharmaceutical and biotechnology companies worldwide. Headquartered in the United States of America and with locations in the USA and Asia, AmbioPharm operates internationally with over 14 years of experience and expertise. Further information is available at: https://www.ambiopharm.com
About YMC:
YMC is a private Life Science company headquartered in Kyoto, Japan. Founded in 1980, YMC has 9 affiliates and several facilities throughout Asia, Europe, and America. Their 500+ employees are providing best-in-class lab and process solutions to the bio/pharmaceutical industry. YMC’s focus is innovation, production, and sales of packing materials, packed columns and systems for High Performance Liquid Chromatography (HPLC), Low Pressure Liquid Chromatography (LPLC), and other custom purification systems. YMC operates a CMO facility in Japan and has recently opened a new lab / pilot facility “Kyoto Works” incorporating state of the art multi-column purification equipment.
YMC America, formerly YMC Process Technologies (YPT) in Devens, MA USA, has supplied GMP scale downstream process system for nearly 20 years. Acquired by YMC in December 2018, YPT Bio/Pharma Systems Group along with its sister affiliate YMC ChromaCon AG (Zurich, Switzerland), is a leading supplier of lab and production scale single and multi-column chromatography systems. YMC’s intellectual assets and know-how, cultivated from many years of experience, will continue to push the limits to create a prosperous future for the production and purification of small and large molecule therapies. More at: https://www.ymcamerica.com/
1MCSGP / Autopeak: US patents 7.837.881, 8.496.836 |
View source version on businesswire.com: https://www.businesswire.com/news/home/20230328005358/en/
Contacts
AmbioPharm
Mark DaFonseca, EVP, Sales & Global Business Development
mark.dafonseca@ambiopharm.com
YMC America
Wayne Nettnay, Global Sales Director
wnettnay@ymcpt.com




